A potent trifluoromethyl ketone histone deacetylase inhibitor exhibits class-dependent mechanism of action
Abstract
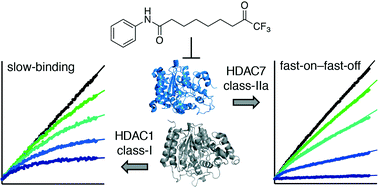
Histone deacetylase (HDAC) enzymes are validated targets for treatment of certain cancers and have potential as targets for pharmacological intervention in a number of other diseases. Thus, inhibitors of these enzymes have received considerable attention, but these are often evaluated by IC50 value determination, which may vary significantly depending on assay conditions. In this work, we therefore performed detailed kinetic evaluation of inhibitors containing two fundamentally different zinc-binding chemotypes, hydroxamic acid or trifluoromethyl ketone. For the hydroxamic acids, a fast-on–fast-off mechanism was observed, but the trifluoromethyl ketone compound exhibited differential mechanisms depending on the enzyme isoform. The trifluoromethyl ketone compound displayed a fast-on–fast-off mechanism against class-IIa HDACs 4 and 7, but slow-binding mechanisms against class-I and class-IIb enzymes (HDACs 1–3, 6 and 8). Furthermore, different competitive, slow-binding mechanisms were observed for HDACs 1, 2, and 6 vs. HDACs 3 and 8, demonstrating the power of kinetic experiments for characterisation of enzyme inhibitors.

 Please wait while we load your content...
Please wait while we load your content...