A mode of action study of cationic anthraquinone analogs: a new class of highly potent anticancer agents†‡
Abstract
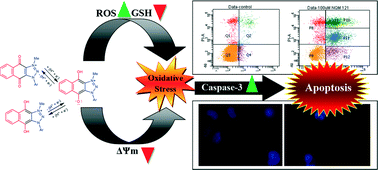
Previously, we reported the synthesis and structure–activity relationship (SAR) study of a series of novel 4,9-dioxo-4,9-dihydro-1H-naphtho[2,3-d][1,2,3]triazol-3-ium salts, which had very potent anti-proliferative activities (low μM to nM GI50) against a broad range of cancer cells. These compounds, which can be viewed as cationic anthraquinone analogs (CAAs), are selective against cancer cells over bacteria or fungi as compared to the antibacterial CAAs that have also been reported by our group. Herein, we report a mode of action study of CAAs, which reveals that these compounds trigger apoptosis by generating extensive reactive oxygen species (ROS). The generation of extensive ROS causes oxidative stress, decrease in mitochondrial membrane potential, depletion of glutathione (GSH), and release of caspase-3, which ultimately kills cancer cells by programmed apoptosis. Furthermore, we have also shown that CAAs possess an 8-fold higher activity against the A549 cell line vs. the non-cancerous MRC-5 cell line.

 Please wait while we load your content...
Please wait while we load your content...