Imidazole derivatives show anticancer potential by inducing apoptosis and cellular senescence†
Abstract
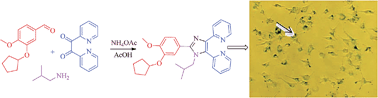
Imidazole-based compounds are attractive targets in the design of novel chemical structures for the discovery of new drugs. In the current study, we have synthesized a series of new 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles by multicomponent reaction (MCR). Vanillin and isovanillin derivatives were reacted with benzil/pyridil and diverse amines and ammonium acetate in acetic acid at 50–110 °C for 24 h to afford respective imidazoles in 55–70% yields. The series of molecules were evaluated for anti-cancer potential against the National Cancer Institute's 60 human cancer cell line panel. Preliminary screening highlighted the anticancer potential of 2,2′-(2-(3-(cyclopentyloxy)-4-methoxyphenyl)-1-isobutyl-1H-imidazole-4,5-diyl)dipyridine (NSC 771432) against different cancer cell types. A549 cells were treated in vitro to determine the mode of action of NSC 771432 on growth of these cells. This compound inhibits anchorage independent growth and cell migration, and induces cell cycle arrest in the G2/M phase. Also, the exposure of A549 cells to NSC 771432 leads to cellular senescence.

 Please wait while we load your content...
Please wait while we load your content...