Synthesis and antimicrobial activities of His(2-aryl)-Arg and Trp-His(2-aryl) classes of dipeptidomimetics†
Abstract
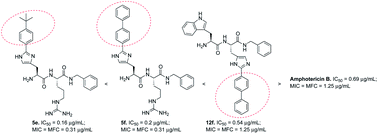
In this communication, we report the design, synthesis and in vitro antimicrobial activity of ultra short peptidomimetics. Besides producing promising antibacterial activities against Staphylococcus aureus and methicillin-resistant S. aureus (MRSA), the dipeptidomimetics exhibited high antifungal activity against C. neoformans with IC50 values in the range of 0.16–19 μg mL−1. The most potent analogs exhibited 4-fold higher activity than the currently used drug amphotericin B, with no apparent cytotoxicity in a panel of mammalian cell lines.

 Please wait while we load your content...
Please wait while we load your content...