Synthesis and antiviral evaluation of 4′-(1,2,3-triazol-1-yl)thymidines†
Abstract
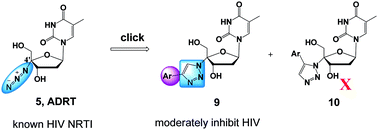
Non-obligate chain terminating nucleosides with a linear substituent (azido or ethynyl group) at the 4′ position represent an important class of compounds in antiviral discovery, particularly against hepatitis C virus (HCV) and human immunodeficiency virus (HIV). We have previously shown that 3′-azidothymidine (AZT)-derived 1,2,3-triazoles can be potent inhibitors of HIV-1. To gauge the medicinal chemistry impact of functionalizing the 4′-linear substituent and possibly generate novel antiviral nucleoside scaffolds, we have explored azide–alkyne cycloaddition reactions with 4′-azidothymidine (ADRT). The Ru-mediated reaction failed and the Cu-catalyzed variant generated 1,2,3-triazoles (9a–y) with only modest yields and efficiencies, indicating a substantial steric barrier around the 4′-azido group. Antiviral screening identified a few triazole analogues moderately active against HIV-1 (18–62% inhibition at 10 μM) and/or influenza A virus (15–50% inhibition at 10 μM), and none active against West Nile virus (WNV) or HCV. These results suggest that the linear 4′ azido group of ADRT may be essential for target binding and that its chemical manipulation could largely compromise antiviral potency.

 Please wait while we load your content...
Please wait while we load your content...