Introduction of polar groups on the naphthalene scaffold of molecular tongs inhibiting wild-type and mutated HIV-1 protease dimerization†
Abstract
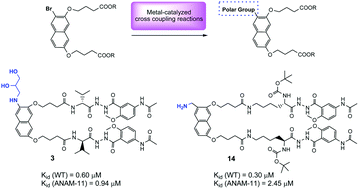
A new series of naphthalene-based molecular tongs containing polar groups at the 3-position of the naphthalene scaffold was synthesized and its anti-dimerization activity was evaluated against HIV-1 protease. The polar groups were introduced mainly via metal-catalysed cross coupling reactions such as the Buchwald–Hartwig amination, the Sonogashira reaction and the Beller cyanation. Kinetic analyses showed that by introducing specific polar substituents on the naphthalene scaffold the inhibitory activity of molecular tongs against wild-type and mutated HIV-1 proteases is maintained, while increasing their water solubility.

 Please wait while we load your content...
Please wait while we load your content...