Design, synthesis and biological evaluation of biphenyl urea derivatives as novel VEGFR-2 inhibitors†
Abstract
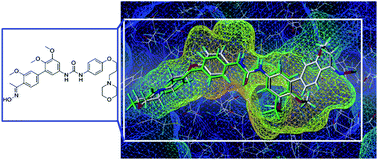
VEGFR-2 plays a critical role in vasculogenesis and VEGFR-2 inhibitors have been widely used in the treatment of cancer. In our continued efforts to search for potent and novel VEGFR-2 inhibitors as antitumor agents, we have identified a potent lead compound (HMQ-16) bearing a biphenyl scaffold. Rearrangement and replacement of arylcarbamoyl in HMQ-16 with a urea moiety generated a series of novel VEGFR-2 inhibitors. In order to enhance the affinity with VEGFR-2, the 4′-acetyl group was converted to an oxime group. Fourteen biphenyl urea derivatives were designed and synthesized as potent VEGFR-2 inhibitors. Six of them (T2, T5, T7, T9, T11, T14) exhibited potent VEGFR-2 inhibitory activity comparable to that of sorafenib. Compound T7 was the most potent with an IC50 value of 1.08 nM. The enzymatic and cellular assays suggested that T7 has potential as a valuable lead compound for further optimization.
 Please wait while we load your content...
Please wait while we load your content...