Molecular modeling studies to characterize N-phenylpyrimidin-2-amine selectivity for CDK2 and CDK4 through 3D-QSAR and molecular dynamics simulations†
Abstract
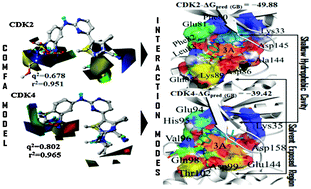
CDK2 is a promising target for the development of anti-cancer agents. It is not an easy task to design CDK2-selective inhibitors which do not exhibit activity for other CDK family members, particularly CDK4, due to a high degree of structural homology among CDK family members. In this study, 4-substituted N-phenylpyrimidin-2-amine derivatives as CDK2 inhibitors were examined to understand the selectivity mechanism against CDK4 using a combined approach of 3D-QSAR, molecular docking, MESP, MD simulations, and binding free energy calculations. 3D-QSAR models were developed to propose structural determinants for CDK2 and CDK4 inhibition. High q2 and r2 values for CoMFA and CoMSIA models based on both internal and external validations suggested that the generated 3D-QSAR models may exhibit good capability to predict bioactivities of inhibitors against CDK2 or CDK4. Electrostatic potentials on the molecular surface have been discussed in detail for determining the binding affinity of studied inhibitors by combining molecular docking with MESP and Mulliken charge analyses. Binding free energy calculations suggested that the residues Gln85, Asp86, and Lys89 of CDK2 would play a critical role in selective CDK2 inhibition. The electrostatic interactions of an inhibitor with Glu144 and Asn145 of CDK4 may predominately drive CDK4 inhibition. These findings may provide a better structural understanding of the mechanism of CDK2 selective inhibition. The results obtained in the current study may provide valuable guidelines for developing novel potent and selective CDK2 inhibitors.

 Please wait while we load your content...
Please wait while we load your content...