l-Asparaginase as a new molecular target against leishmaniasis: insights into the mechanism of action and structure-based inhibitor design†
Abstract
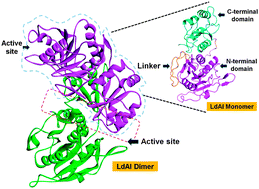
L-Asparaginases belong to a family of amidohydrolases that catalyze the conversion of L-asparagine into L-aspartic acid and ammonia. Although bacterial L-asparaginases have been used extensively as anti-leukemic agents, their possible role as potential drug targets for pathogenic organisms has not been explored. The presence of genes coding for putative L-asparaginase enzymes in the Leishmania donovani genome hinted towards the specific role of these enzymes in extending survival benefit to the organism. To investigate whether this enzyme can serve as a potential drug target against the Leishmania pathogen, we obtained structural models of one of the putative Leishmania L-asparaginase I (LdAI). Using an integrated computational approach involving molecular modelling, docking and molecular dynamics simulations, we found crucial differences between catalytic residues of LdAI as compared to bacterial L-asparaginases. The deviation from the canonical acid–base pair at triad I, along with the structural reorganization of a β-hairpin loop in the presence of a substrate, indicated an altogether new mechanism of action of the LdAI enzyme. Moreover, the finding of compositional and functional differences between LdAI and human asparaginase was used as a criterion to identify specific small molecule inhibitors. Through virtual screening of a library of 11 438 compounds, we report five compounds that showed favorable interactions with the active pocket of LdAI, without adversely affecting human asparaginase. One of these compounds when tested on cultured Leishmania promastigotes displayed a promising leishmanicidal effect. Overall, our work not only provides first hand mechanistic insights of LdAI but also proposes five strongly active compounds which may prove as effective anti-leishmaniasis molecules.

 Please wait while we load your content...
Please wait while we load your content...