How does conformational flexibility influence key structural features involved in activation of anaplastic lymphoma kinase?†
Abstract
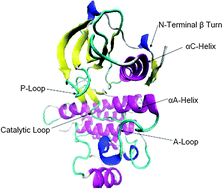
Anaplastic Lymphoma Kinase (ALK) plays a major role in developing tumor processes and therefore has emerged as a validated therapeutic target. Applying atomistic molecular dynamics simulations on the wild type enzyme and the nine most frequently occurring and clinically important activation mutants we revealed important conformational effects on key interactions responsible for the activation of the enzyme.

 Please wait while we load your content...
Please wait while we load your content...