Computational investigation of theoretical models of cleavable and uncleavable mucin 1 isoforms
Abstract
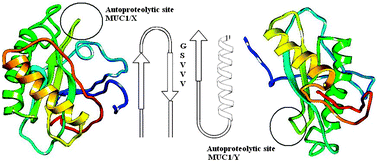
Mucin 1 is an important tumor marker, and is notable for the autoproteolytic event, a signature of all SEA domain containing proteins. However the isoform MUC1/Y, unlike its closest counterpart MUC1/X, is noted to be uncleavable despite the presence of the catalytic site. Sequence analysis has revealed the presence of an 18 residue segment spliced out from MUC1/Y marking the only difference between uncleavable MUC1/Y and cleavable MUC1/X mucin 1 isoforms. This work was aimed at studying in silico, the structural and functional significance of the 18 residue insert in the cleavage process. 3-Dimensional structures of the isoforms were predicted using a combined threading and ab initio method followed by mutation and normal mode analysis. Our analysis revealed both isoforms to poses an intact SEA domain, nevertheless, an altered functional scaffold around the cleavage site was noted between the structures. Mutation analysis by alanine substitutions at the insert region revealed Ile67 to be more destabilizing, resulting in increased fluctuation of the neighboring residues. Predicted molecular motions of the protein indicated localized motions in the native, rather than the mutated model. Intramolecular interactions between the native and I67A structures showed wide variations in main chain and side chain interactions. Furthermore, the findings suggest the neighboring residues, in particular Ile67 contribute more to the structural and functional properties of the protein and hence it is predicted to be one of the crucial residues for the cleavable nature of MUC1/X.

 Please wait while we load your content...
Please wait while we load your content...