Target analysis of α-alkylidene-γ-butyrolactones in uropathogenic E. coli†
Abstract
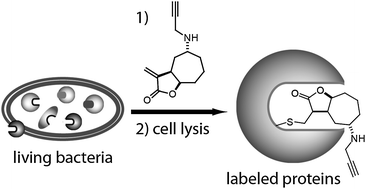
α-Alkylidene-γ-butyrolactones are quite common in nature and exhibit a broad spectrum of biological activities. We therefore synthesized a small library of xanthatine inspired α-alkylidene-γ-butyrolactones to screen non-pathogenic and uropathogenic E. coli strains by activity based
 Please wait while we load your content...
Please wait while we load your content...