Crystal structure of a dimeric Ser49 PLA2-like myotoxic component of the Vipera ammodytes meridionalis venomics reveals determinants of myotoxicity and membrane damaging activity
Abstract
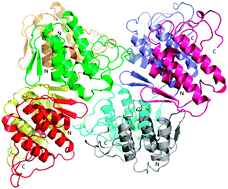
Myotoxicity and membrane damage play a central role in the life-threatening effects of the viper envenomation. Myotoxins are an important part of the viper venomics. A Ser49 PLA2-like myotoxin from the venom of Vipera ammodytes meridionalis, the most venomous snake in Europe, was crystallized and its three-dimensional structure determined. The

 Please wait while we load your content...
Please wait while we load your content...