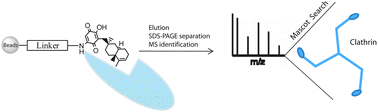
The emerging field of mass spectrometry-based chemical proteomics provides a powerful instrument in the target discovery of bioactive small-molecules, such as drugs or natural products. The identification of their macromolecular targets is required for a comprehensive understanding of their bio-pharmacological role and for unraveling their mechanism of action. We report the application of a chemical proteomics approach to the analysis of the cellular interactome of the marine metabolite bolinaquinone (BLQ). BLQ was linked to an opportune α,ω-diamino polyethylene glycol chain and then immobilized on a matrix support. The modified beads were then used as a bait for fishing the potential partners of BLQ in a THP-1 macrophage cell lysate. Surprisingly, we identified clathrin, a protein involved in the cell internalization of proteins, viruses and other biologically relevant macromolecules, as a specific and major BLQ partner. In addition, we verified the biochemical role of BLQ testing its ability to inhibit the clathrin-mediated endocytosis of albumin. This finding indicates BLQ as a new biotechnological tool for cell endocytosis studies and paves the way to further investigation on its potential role in modulating internalization process.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...