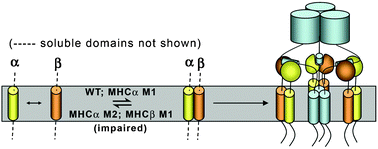
The Major Histocompatibility Complex Class II (Class II MHC) and invariant chain (Ii) proteins are key initiators of an immune response to invading pathogens. Following biosynthesis, three MHCα/β hetero-dimers associate with an Ii homotrimer to form a nine-chain protein complex. Only as part of this complex are the MHC molecules exported to the cell surface to trigger an immune response. Previous reports implicate the transmembrane (TM) domains of all three proteins in correct assembly, ligand binding and function of Class II MHC. Building on our previous work that revealed the Ii TM domain may contribute significantly to correct assembly of the full-length protein, we have used a variety of genetic, biophysical and computational methods to investigate the role of the TM domains in stabilizing MHCα/β heterodimers. Using the in vivo GALLEX assay, we find that the TM domains of both proteins form strong homo- and hetero-oligomers in natural membranes that are stabilized by GXXXG motifs within the sequence. Förster resonance energy transfer (FRET) measurements, using fluorescently-tagged peptides derived from the TM domains of each protein, were then employed to confirm the presence of TM helix–helix hetero-interactions in detergent micelles, as well as the stoichiometry of these interactions. Our results are summarized in a revised model of Class II MHC–Ii complex formation that illustrates key protein–protein contacts. This work provides the first evidence that the TM domains of the Class II MHC molecules are capable of significant protein–protein interactions that may help to stabilize or even initiate formation of the MHC–Ii complex.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...