Temporally resolved direct delivery of second messengers into cells using nanostraws†
Abstract
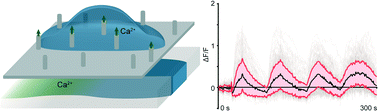
Second messengers are biomolecules with the critical role of conveying information to intracellular targets. They are typically membrane-impermeable and only enter cells through tightly regulated transporters. Current methods for manipulating second messengers in cells require preparation of modified cell lines or significant disruptions in cell function, especially at the cell membrane. Here we demonstrate that 100 nm diameter ‘nanostraws’ penetrate the cell membrane to directly modulate second messenger concentrations within cells. Nanostraws are hollow vertical nanowires that provide a fluidic conduit into cells to allow time-resolved delivery of the signaling ion Ca2+ without chemical permeabilization or genetic modification, minimizing cell perturbation. By integrating the nanostraw platform into a microfluidic device, we demonstrate coordinated delivery of Ca2+ ions into hundreds of cells at the time scale of several seconds with the ability to deliver complex signal patterns, such as oscillations over time. The diffusive nature of nanostraw delivery gives the platform unique versatility, opening the possibility for time-resolved delivery of any freely diffusing molecules.


 Please wait while we load your content...
Please wait while we load your content...