Hydrogen peroxide concentration by pervaporation of a ternary liquid solution in microfluidics†
Abstract
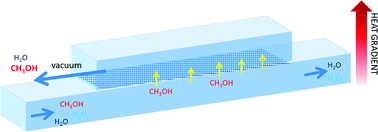
Pervaporation in a microfluidic device is performed on liquid ternary solutions of hydrogen peroxide–water–methanol in order to concentrate hydrogen peroxide (H2O2) by removing methanol. The quantitative analysis of the pervaporation of solutions with different initial compositions is performed, varying the operating temperature of the microfluidic device. Experimental results together with a mathematical model of the separation process are used to understand the effect of the operating conditions on the microfluidic device efficiency. The parameters influencing significantly the performance of pervaporation in the microfluidic device are determined and the limitations of the process are discussed. For the analysed system, the operating temperature of the chip has to be below the temperature at which H2O2 decomposes. Therefore, the choice of an adequate reduced operating pressure is required, depending on the expected separation efficiency.
 Please wait while we load your content...
Please wait while we load your content...