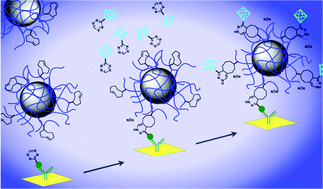
Efficient methods to immobilize small molecules under continuous-flow microfluidic conditions would greatly improve label-free molecular interaction studies using biosensor technology. At present, small-molecule immobilization chemistries require special conditions and in many cases must be performed outside the detector and microfluidic system where real-time monitoring is not possible. Here, we have developed and optimized a method for on-chip bioorthogonal chemistry that enables rapid, reversible immobilization of small molecules with control over orientation and immobilization density, and apply this technique to surface plasmon resonance (SPR) studies. Immobilized small molecules reverse the orientation of canonical SPR interaction studies, and also enable a variety of new SPR applications including on-chip assembly and interaction studies of multicomponent structures, such as functionalized nanoparticles, and measurement of bioorthogonal reaction rates. We use this approach to demonstrate that on-chip assembled functionalized nanoparticles show a preserved ability to interact with their target protein, and to measure rapid bioorthogonal reaction rates with k2 > 103 M−1 s−1. This method offers multiple benefits for microfluidic biological applications, including rapid screening of targeted nanoparticles with vastly decreased nanoparticle synthetic requirements, robust immobilization chemistry in the presence of serum, and a continuous flow technique that mimics biologic contexts better than current methods used to measure bioorthogonal reaction kinetics such as NMR or UV-vis spectroscopy (e.g., stopped flow kinetics). Taken together, this approach constitutes a flexible and powerful technique for evaluating a wide variety of reactions and intermolecular interactions for in vitro or in vivo applications.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...