Activated T lymphocytes migrate toward the cathode of DC electric fields in microfluidic devices†
Abstract
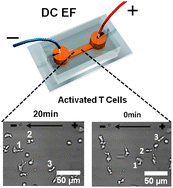
Immune cell migration is a fundamental process that enables immunosurveillance and immune responses. Understanding the mechanism of immune cell migration is not only of importance to the biology of cells, but also has high relevance to cell trafficking mediated physiological processes and diseases such as embryogenesis, wound healing, autoimmune diseases and cancers. In addition to the well-known chemical concentration gradient based guiding mechanism (i.e.chemotaxis), recent studies have shown that lymphocytes can respond to applied physiologically relevant direct current (DC) electric fields by migrating toward the cathode of the fields (i.e. electrotaxis) in both in vitro and in vivo settings. In the present study, we employed two microfluidic devices allowing controlled application of electric fields inside the microfluidic channel for quantitative studies of lymphocyte electrotaxis in vitro at the single cell level. The first device is fabricated by soft-

 Please wait while we load your content...
Please wait while we load your content...