Organic/inorganic hybrid electrochromic devices based on photoelectrochemically formed polypyrrole/TiO2 nanohybrid films†
Abstract
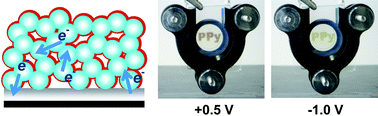
Electrochromic devices employing photoelectrochemically fabricated polypyrrole/TiO2 nanohybrid films have been demonstrated for the first time. The nanohybrid film was successfully formed photoelectrochemically by exciting nanocrystalline TiO2 with UV light irradiation in the presence of pyrrole molecules in an electrochemical cell. The nanohybrid film exhibited electrochromic colour changes similar to the flat polypyrrole film. The fastest polypyrrole reduction response of 0.4 ms (evaluated by an absorbance change from 0 to 90%) was achieved. The electrochromic responses of the hybrid films were controlled by relative potential energy levels between the TiO2 conduction band edge and the polypyrrole redox potential, adjusted by the solution pH, and homogeneity/thickness of a polypyrrole layer on the surface of a TiO2 film. Based on the experimental results, the dual electron transport mechanisms influencing the electrochromic behaviours are suggested: Path 1, electron transport along the polypyrrole layer; and Path 2, electron transport through the nanocrystalline TiO2 conduction band and electron transfer reactions at the hybrid interface. Finally, the pattern electrochromic reactions can now be implemented as the direct application to display devices.

 Please wait while we load your content...
Please wait while we load your content...