Electrochemically stimulated release of lysozyme from an alginate matrix cross-linked with iron cations†
Abstract
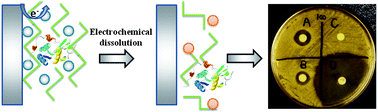
An electrochemically generated alginate matrix cross-linked with Fe3+
- This article is part of the themed collection: Materials for biosurfaces

 Please wait while we load your content...
Please wait while we load your content...