Magnesium hydride as a high capacity negative electrode for lithium ion batteries†
Abstract
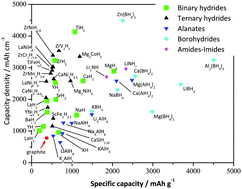
Conversion reactions in lithium batteries have been proved for several classes of materials, such as oxides, fluorides, sulphides, nitrides, phosphides and recently for hydrides. Metal hydrides can be electrochemically reduced to a highly conductive composite material consisting of nanometric metallic particles dispersed in an amorphous LiH matrix. Magnesium hydride undergoes a reversible conversion reaction and it has very good theoretical performances, i.e. a theoretical specific capacity of 2038 mA h g−1 and a working potential of 0.5 V vs. Li+/Li. The purpose of our study is to investigate the MgH2 redox activity by evaluating the effect of ball milling pre-treatments and by studying the conversion reaction mechanism. Three materials, prepared by submitting bulk MgH2 to different ball milling procedures, are investigated. By coupling electrochemical tests, ex situ X-ray powder diffraction and transmission electron microscopy, we prove that the lithium incorporation does not follow a simple direct conversion path as it follows at least a sequence of four consecutive processes: (a) the hydride conversion reaction of MgH2 to give Mg and LiH, (b) the alloying of Li in hcp Mg and (c and d) the formation and lithium enrichment of a bcc Li–Mg solid solution. Furthermore some experimental clues suggest that the mechanism is probably even more complex as it can imply the formation of other unknown intermediate Li–Mg–H phases. Moreover large morphological changes occur upon lithium incorporation in the electrodes: in particular an extended sintering of the metal nanoparticles occurs upon cycling. This effect leads to electrode pulverization and capacity fading. On the other hand MgH2 shows a very limited potential hysteresis between discharge and charge and very promising kinetics at high current.

 Please wait while we load your content...
Please wait while we load your content...