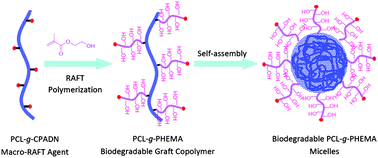
Biodegradable micelles were prepared from poly(ε-caprolactone)-g-poly(2-hydroxyethyl methacrylate) (PCL-g-PHEMA) graft copolymers and investigated for controlled release of doxorubicin (DOX). PCL-g-PHEMA copolymers were readily obtained by controlled ring-opening copolymerization of acryloyl cyclic carbonate and ε-caprolactone, Michael-type conjugate addition reaction with cysteamine, coupling reaction with 4-cyanopentanoic acid dithionaphthalenoate (CPADN) via carbodiimide chemistry, and reversible addition–fragmentation chain transfer (RAFT) polymerization of 2-hydroxyethyl methacrylate (HEMA). 1H NMR analyses showed that Mn of PHEMA ranged from 8.7, 16.3 to 33.8 kg mol−1, in proximity to the design as well as those determined by gel permeation chromatography (GPC). Differential scanning calorimetry (DSC) revealed that all three PCL-g-PHEMA graft copolymers had depressed melting temperatures (Tm = 31.3–32.5 °C) and low crystallinities (Xc = 3.05–5.66%). Dynamic light scattering (DLS) showed that PCL-g-PHEMA formed monodisperse micelles with low polydispersity indexes of 0.04–0.16 and average sizes ranging from 80.5 to 179.7 nm depending on PHEMA chain lengths. These graft copolymers displayed low critical micelle concentrations (CMCs) of 0.051–0.151 μM. The micellar sizes decreased following loading with DOX while PDI remained low. Interestingly, in vitro drug release studies showed that DOX-loaded PCL-g-PHEMA micelles exhibited superior pH-responsive release behaviors, in which up to 94.5% of DOX was released in 3 d at pH 5.0 while DOX release was significantly slower at pH 7.4 (maximum 54.1% release in 3 d). MTT assays with HeLa cells demonstrated that DOX-loaded PCL-g-PHEMA micelles retained high anti-tumor activity with low IC50 (half inhibitory concentration) of 1.47–1.74 μg DOX equiv. mL−1 while PCL-g-PHEMA micelles were practically non-toxic up to a tested concentration of 80 mg mL−1. These novel biodegradable PCL-g-PHEMA graft copolymer micelles with low CMC, small and tunable sizes, high drug loading, and pH-responsive drug release have emerged as superior nanocarriers for “smart” tumor-targeting drug delivery.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...