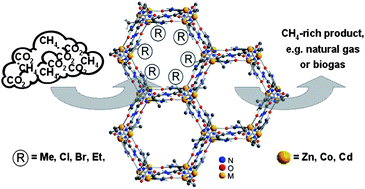
In this work the adsorption of CO2 and CH4 on a series of isoreticular microporous metal–organic frameworks based on 2-substituted imidazolate-4-amide-5-imidates, IFP-1–IFP-6 (IFP = Imidazolate Framework Potsdam), is studied firstly by pure gas adsorption at 273 K. All experimental isotherms can be nicely described by using the Tòth isotherm model and show the preferred adsorption of CO2 over CH4. At low pressures the Tòth isotherm equation exhibits a Henry region, wherefore Henry's law constants for CO2 and CH4 uptake could be determined and ideal selectivity αCO2/CH4 has been calculated. Secondly, selectivities were calculated from mixture data by using nearly equimolar binary mixtures of both gases by a volumetric–chromatographic method to examine the IFPs. Results showed the reliability of the selectivity calculation. Values of αCO2/CH4 around 7.5 for IFP-5 indicate that this material shows much better selectivities than IFP-1, IFP-2, IFP-3, IFP-4 and IFP-6 with slightly lower selectivity αCO2/CH4 = 4–6. The preferred adsorption of CO2 over CH4 especially of IFP-5 and IFP-4 makes these materials suitable for gas separation application.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...