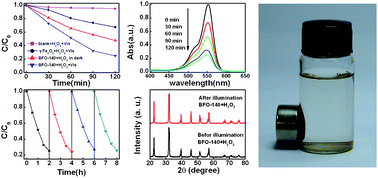
A facile sol–gel approach with a fixed calcination temperature is developed to prepare BiFeO3 (BFO) nanoparticles, and the gel-drying temperature is adjusted to control the appearance of a γ-Fe2O3 parasitic phase. The room temperature ferromagnetism of the samples is strongly dependent on the gel-drying temperature. When the gel-drying temperature increases from 80 to 140 °C, the saturated magnetization of the corresponding samples jumps from 0.22 emu g−1 to 1.2 emu g−1, allowing the nanoparticles to be magnetically separated in solution. From examination by transmission electron microscopy and X-ray photoelectron spectroscopy, it is confirmed that the γ-Fe2O3 parasitic phase is nucleated during the gel-drying process under high temperatures above 120 °C, and remains in the subsequent annealing process, resulting in the anomalous enhanced magnetization. Comparing with pure BFO nanoparticles prepared under low gel-drying temperature, the BFO/γ-Fe2O3 samples exhibit significantly increased visible-light photocatalytic ability towards rhodamine B. The formation of a heterojunction structure between the BFO and γ-Fe2O3 phases is proposed to be responsible for the enhanced photocatalytic activity. Further enhanced photocatalytic activity is obtained in this study when adding a small amount of H2O2 during photocatalysis, indicating the samples have photo-Fenton-like catalytic activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...