Formation and functionalization of surface Diels–Alder adducts on ethenylene-bridged periodic mesoporous organosilica†
Abstract
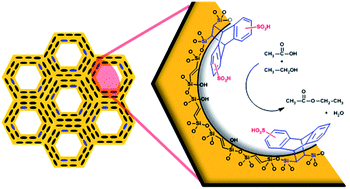
The Diels–Alder cycloaddition of two common dienes (cyclopentadiene and anthracene) to the double carbon–carbon bonds of an ethenylene-bridged periodic mesoporous organosilica was studied and compared to that of benzocyclobutene. The resulting materials were characterized by several techniques such as X-ray and thermal analyses, DRIFT, 13C and 29Si MAS NMR and porosimetry. They showed that the mesopores were decorated with the Diels–Alder adducts with negligible structural degradation and with the concomitant reduction of the surface area and pore size. The formation of surface adducts was even successful with a relatively hindered diene such as anthracene. The surface Diels–Alder adducts were stable and susceptible to further functionalization. Thus, after being sulfonated, the resulting solids were used as acid catalysts in the esterification of acetic acid with ethanol, a process in which they proved to be very active. In fact, each acid site of these hybrid materials was up to three times more active than a similar site in Amberlyst-15, a commercial acid resin.

 Please wait while we load your content...
Please wait while we load your content...