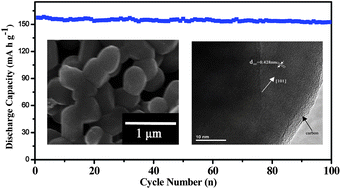
From the viewpoint of energy efficiency and cost, an intensive search for less energy demanding synthesis methods for LiFePO4 appears quite attractive and is still in full swing. Here, we report that carbon coated LiFePO4 (LiFePO4/C) powders can be successfully synthesized by a new route using supercritical carbon dioxide as a solvent. The obtained high-purity LiFePO4/C powders are uniform in shape and 0.5 µm to 1 µm in size. As for the electrochemical performance, at the current density of 0.1 C, the electrode exhibits a discharge capacity of 158 mA h g−1 as well as good cycling stability—there is no obvious capacity fading after 100 cycles. When the synthesis reaction temperature increases from 50 to 200 °C, the primary particle size grows from approximately 0.5 to 2 µm, and the initial discharge capacity decreases from 158 to 41 mA h g−1, which shows that 50 °C is the optimum temperature to synthesize LiFePO4 in supercritical carbon dioxide. Needless to say, such a new approach, which is not specific to LiFePO4, offers great opportunities for the synthesis of new electrode materials.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...