Improved kinetics and stabilities in Mg-substituted LiMnPO4
Abstract
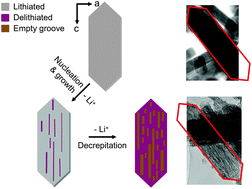
LiMgxMn1−xPO4 (x = 0, 0.1, 0.2, 0.3, 0.4 and 0.5) crystals were prepared hydrothermally. The presence of Mg2+ was found to improve the kinetics, utilization, and physical stability of the crystals during chemical and electrochemical delithiation, as well as the thermal stability of the delithiated phase. The best performance was found in the sample with 20% substitution. The positive effect of Mg2+ was attributed to the reduced volume mismatch between the lithiated and delithiated phases, and to more favorable particle morphologies. Mg2+ dilutes the concentration of Jahn–Teller active ion, Mn3+, and reduces local strains between the phases, and thereby increases the structural stability of the crystals. The result is a reduction in fracturing and decrepitation, which translates to improved electrochemical performance. Although the thermal stability improved with increasing Mg substitution, the heat evolved during reaction with electrolyte remains proportional to the Mn content and therefore to the theoretical capacity.
- This article is part of the themed collection: Advanced Materials for Lithium Batteries

 Please wait while we load your content...
Please wait while we load your content...