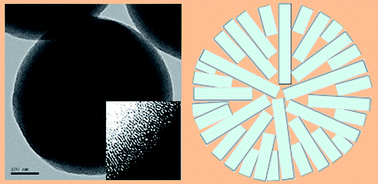
Hydrothermally highly stable mesoporous aluminosilicate spheres with radial channels were synthesized in the CTAB–NaF–TPAOH system through a one-step procedure at high aging temperature. The characterization by transmission electron microscopy (TEM), X-ray diffraction (XRD), nitrogen adsorption/desorption analysis, 27Al MAS solid state NMR spectroscopy, pyridine adsorption FT-IR combined with the typical hydrothermal treatments showed that this kind of material exhibited large surface area, specific pore arrangement, strong acidity and high hydrothermal stability. Detailed studies suggest that F− ions direct the perpendicular arrangement of aluminosilicate clusters during the hydrothermal treatment at 160 °C, while TPA+ stabilized the structure. Both F− and TPA+ ions are considered to improve the acidity and hydrothermal stability of this material through coordination of framework atoms, thus, enhancing the condensation of Si–O–Si bonds in the amorphous pore walls. Due to the accessible radial pore arrangement and high acidity, the catalytic activity for Friedel–Crafts alkylation of toluene with benzyl alcohol was excellent with 100% conversion of benzyl alcohol.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...