Visible light response of AgLi1/3M2/3O2 (M = Ti and Sn) synthesized from layered Li2MO3 using molten AgNO3
Abstract
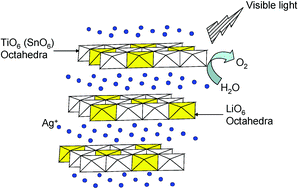
AgLi1/3Ti2/3O2 and AgLi1/3Sn2/3O2 with delafossite structures were synthesized by treating the layered compounds Li2TiO3 and Li2SnO3 with molten AgNO3 (573 K for 5 h) through ion exchange of Li+ for Ag+. The band gaps of both AgLi1/3Ti2/3O2 and AgLi1/3Sn2/3O2 were estimated to be 2.7 eV from

 Please wait while we load your content...
Please wait while we load your content...