Origin of the large spectral shift in electroluminescence in a blue light emitting cationic iridium(iii) complex
Abstract
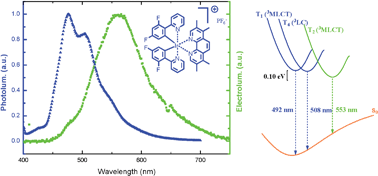
A new, but archetypal compound [Ir(ppy-F2)2Me4phen]PF6, where ppy-F2 is 2-(2′,4′-fluorophenyl)pyridine and Me4phen is 3,4,7,8-tetramethyl-1,10-phenanthroline, was synthesized and used to prepare a solid-state light-emitting electrochemical cell (LEEC). This complex emits blue light with a maximum at 476 nm when photoexcited in a thin film, with a photoluminescence quantum yield of 52%. It yields an efficient single-component solid-state electroluminescence device with a current efficiency reaching 5.5 cd A−1 and a maximum power efficiency of 5.8 Lm Watt−1. However, the electroluminescence spectrum is shifted with respect to the photoluminescence spectrum by 80 nm resulting in the emission of green light. We demonstrate that this unexpected shift in emission spectrum does not originate from the mode of excitation, nor from the presence of large concentrations of ions, but is related to the concentration of the ionic transition metal complex in the thin film. The origin of the concentration-dependent emission is extensively commented on and argued to be related to the population of either 3LC π–π* or 3MLCT triplet states, in diluted and concentrated films, respectively. Using quantum chemical calculations we demonstrate that three low-energy triplet states are present with only 0.1 eV difference in energy and that their associated emission wavelengths differ by as much as 60 nm from each other.

 Please wait while we load your content...
Please wait while we load your content...