Abstract
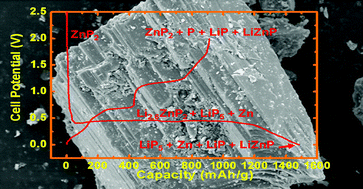
Monoclinic ZnP2 particles were synthesized by vacuum  000 °C. In contrast to a tetragonal Zn3P2 phase, a very large plateau corresponding to 1
000 °C. In contrast to a tetragonal Zn3P2 phase, a very large plateau corresponding to 1 000 mAh g−1 at ∼0.45 V was developed, and out to 545 mAh g−1, only topotactic lithium ion intercalation into the molecule pores was observed. The excess Li ion uptake beyond simple Li intercalation (>545 mAh g−1) into molecular pores can break a chemical bond between Zn and the phosphorus atoms. During discharge, the formation of the LinP clusters (LiP5 and LiP) and Zn, LiZnP phases were dominant as a result of the local structural distortion around the ZnP4 tetrahedral site. During charge, Zn and LiP5 phases transformed into ZnP2 and LiP phases. However, decomposition reactions of the LiP and ZnP2 phases with the electrolyte led to the capacity fade of the cell.
000 mAh g−1 at ∼0.45 V was developed, and out to 545 mAh g−1, only topotactic lithium ion intercalation into the molecule pores was observed. The excess Li ion uptake beyond simple Li intercalation (>545 mAh g−1) into molecular pores can break a chemical bond between Zn and the phosphorus atoms. During discharge, the formation of the LinP clusters (LiP5 and LiP) and Zn, LiZnP phases were dominant as a result of the local structural distortion around the ZnP4 tetrahedral site. During charge, Zn and LiP5 phases transformed into ZnP2 and LiP phases. However, decomposition reactions of the LiP and ZnP2 phases with the electrolyte led to the capacity fade of the cell.
- This article is part of the themed collection: New energy materials

 Please wait while we load your content...
Please wait while we load your content...