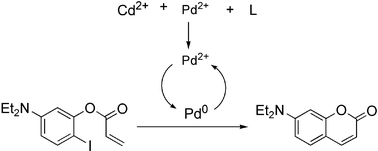
This paper describes a new method for heavy metal analysis via catalytic signal amplification. This signal amplification protocol relies upon an exogenous inhibitor for deliberate deactivation of an organometallic reaction that catalytically creates a fluorophore. When the deactivation process is performed in the presence of the analyte, competitive binding of the inhibitor with the analyte and the catalyst occurs. A Heck reaction creating a coumarin fluorophore with a high quantum yield was studied. 1,4,7,10,13-Pentaaza-cyclopentadecane was chosen as the inhibiting ligand to selectively coordinate pre-catalyst Pd(II) and analytes Cd(II), Ni(II), Co(II). Monitoring product fluorescent intensity in real time revealed the concentration of analyte.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...