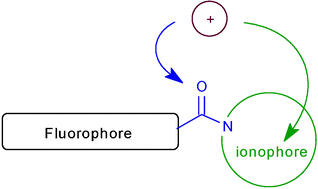
About the use of an amide group as a linker in fluoroionophores: competition between linker and ionophore acting as chelating groups
Abstract
The photophysical and complexing properties of a series of aza-
- This article is part of the themed collection: Fluorescent sensors

 Please wait while we load your content...
Please wait while we load your content...