Chemical composition and crystal structure of superconducting sodium cobalt oxide bilayer-hydrate†
Abstract
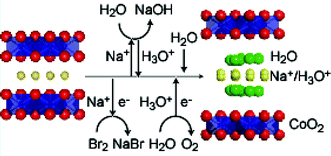
Sodium cobalt oxide bilayer-hydrate, NaxCoO2·yH2O (x ≈ 0.35), was characterized by chemical and structure analyses. The formal valence of Co was found to be +3.4 from redox titration, which was much lower than +3.65 that had been estimated from the Na content, x. The deviation from the charge neutralization was considered to be compensated by the presence of oxonium ions in the CoO2 galleries, which was evidenced by Raman spectroscopy. Therefore, the composition of the superconducting phase was determined to be Na0.343(H3O)0.237CoO2·1.19H2O. Structural studies based on Raman spectroscopy and synchrotron X-ray powder diffraction suggested that the oxonium ions occupy the same crystallographic sites as the Na+ ions.

 Please wait while we load your content...
Please wait while we load your content...