New alkaline earth substituted lithium trivanadates: synthesis, characterization and lithium insertion behavior
Abstract
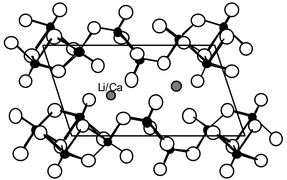
New Mg, Ca and Ba partially substituted materials were prepared by ion exchange of Li+ in a Li1.1V3O8 precursor annealed at 350 °C. They present the same morphology as the precursor, with no well defined crystal shape. The chemical analyses lead to the general formula Li1.1−2yMyHzV3O8
(M = Mg, Ca, Ba; y
≈ 0.05–0.2; z
≈ 0.1). X-Ray diffraction analyses including Rietveld refinements have shown that the structure is basically the same as in the precursor. After the ion exchange, the materials present a larger surface area and a slightly smaller interlayer spacing. The Li insertion behaviors of the substituted materials and of the Li1.1V3O8 precursor present great similarities, confirmed by detailed studies using various galvanostatic or potentiodynamic techniques. Even if they present smaller initial capacities, the exchanged compounds exhibit both enhanced cycling capacities and decreased relative capacity losses on cycling. The diminution of relative capacity loss on cycling is believed to come from a structural stabilization by the divalent

 Please wait while we load your content...
Please wait while we load your content...