The one dimensional chain structures of vanadyl glycolate and vanadyl acetate†
Abstract
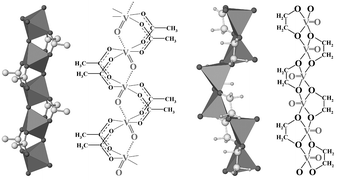
The ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of 1.684(7)
Å and a short trans V-O bond of 2.131(7)
Å, and by bridging acetate groups. The vanadium atoms interact along the ⋯V
O bond of 1.684(7)
Å and a short trans V-O bond of 2.131(7)
Å, and by bridging acetate groups. The vanadium atoms interact along the ⋯V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯V
O⋯V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯ chain giving one-dimensional antiferromagnetic behavior. In contrast in the glycolate, the apical V
O⋯ chain giving one-dimensional antiferromagnetic behavior. In contrast in the glycolate, the apical V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond is shorter, 1.58(1)
Å, and the square pyramids share edges in a two up–two down fashion to give chains of formula VO(OCH2CH2O). Magnetic susceptibility of vanadyl glycolate is consistent with an isolated spin dimers model.
O bond is shorter, 1.58(1)
Å, and the square pyramids share edges in a two up–two down fashion to give chains of formula VO(OCH2CH2O). Magnetic susceptibility of vanadyl glycolate is consistent with an isolated spin dimers model.

 Please wait while we load your content...
Please wait while we load your content...