Layered nickel hydroxide salts Ni(OH)2−x(A)x·nH2O (A = NO3−, n-alkylsulfonates CnH2n+1SO3− with n
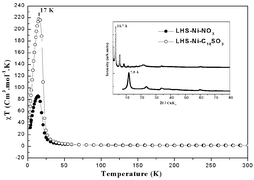
= 10, 14, 18) have been prepared by exchange reaction in aqueous medium at pH = 8.5, starting from the layered hydroxide acetate Ni(OH)1.5(CH3COO)0.5·nH2O. This parent compound has been synthesized by hydrolysis in polyol medium. The obtained compounds present the typical features of the brucite-like structure with turbostratic disorder and an interlayer spacing varying in the range 7.5–32 Å. The magnetic properties have been investigated by means of dc and ac measurements. Two main interactions are found in these materials. On one hand, ferromagnetic in-plane exchange interactions dominate at high temperature, as deduced from the positive Weiss constant, while interlayer ferromagnetic interactions of dipolar origin are responsible for the 3D order at low temperature. This order is indicated by the hysteresis loops below a critical temperature lying in the range 16–18 K. The magnetic properties will be discussed in relation to the structural features, and are mainly driven by the length of the intercalated anion and its head-group functionality. It will be shown that these compounds form a novel series of layered materials for which the model of ferromagnetic layers interacting through dipolar coupling can be successfully applied.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...