Abstract
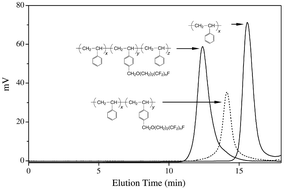
New narrow polydisperse polystyrene-based block copolymers comprising fluorinated aromatic substituents in the side chains were synthesized following two different synthetic routes: (i) TEMPO-mediated controlled radical polymerization of fluorinated styrene monomers; (ii) A sequence of polymer modification reactions on anionically formed polystyrene-block-polyisoprene block copolymers. Using the former route, AB diblock and ABA triblock copolymers were obtained in which a block of polystyrene bearing a fluorocarbon chain substituent with a range of nCF2 groups (n = 4, 6, or 8) was incorporated as B block. Following the latter route, polystyrene-block-polyisoprene AB diblock copolymers were prepared by anionic polymerization and then modification by introduction of fluorinated tails with different numbers of CF2 groups (n = 6 or 8). The thermal behavior as well as the bulk microstructure of the fluorinated block copolymers were investigated and the effect of the chemical structure on the properties was evaluated. All the samples showed a tendency to form layered mesophases in the bulk, and increasing the length of the fluorocarbon tail of the side chain enhanced the degree of order of the mesophase from a disordered smectic (n = 4 or 6) to ordered pseudohexagonal smectic (n = 8). Contact angles of block copolymer films were measured using water as the wetting medium. Values of advanced contact angles up to 130° (120° receded angles) were found which were quite constant over prolonged immersion times in water.

 Please wait while we load your content...
Please wait while we load your content...