Abstract
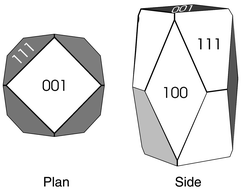
Computer modelling techniques are used to investigate the surface properties and defect chemistry of the La2NiO4 material. Relaxed surface structures and energies are calculated for the low index planes which are used to predict the equilibrium crystal morphology. The {111} surface is calculated to dominate in the absence of impurities,

 Please wait while we load your content...
Please wait while we load your content...