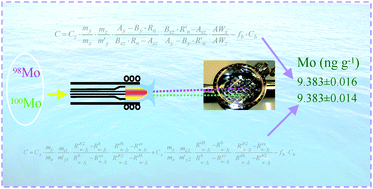
Two isotope dilution calibration approaches, a standard two-step and a curve method, were examined in an effort to achieve highest accuracy and precision for determination of Mo in seawater. On-line matrix separation using immobilized 8-hydroxyquinoline permitted transient measurements to be made by both sector field sequential (SF-ICP-MS) and multicollector (MC-ICP-MS) inductively coupled plasma mass spectrometry. Concentrations of 9.42 ± 0.22 and 9.396 ± 0.053 ng g−1Mo (U, 95% confidence interval, k = 3.18, n = 4) were obtained in NASS-5 seawater using the standard two-step ID method with SF-ICP-MS and MC-ICP-MS, respectively, in good agreement with the certified value of 9.4 ± 1.0 ng g−1 (U, 95% confidence interval, k = 2; concentration converted from ng ml−1 to ng g−1). A 4-fold enhancement in the precision of determination was evident using MC-ICP-MS. Performance of the standard two-step ID and the ID curve method was also compared following an off-line column separation which permitted steady-state solution nebulization with MC-ICP-MS detection. Undertaking mass bias drift correction during a measurement sequence generated identical concentrations of 9.383 ± 0.016 and 9.383 ± 0.014 ng g−1Mo (U, 95% confidence interval, k = 3.18, n = 4) in NASS-5 using the two-step ID and the ID curve method, respectively, despite significant blank correction arising from the off-line column separation which amounted to 4–5% of the total Mo in the sample. Although the ID curve method uses an additional set of reverse ID calibration solutions, it benefits from the advantages of not needing to employ a certified isotopic reference material for mass bias correction or to know isotope abundances of the analyte in the sample, the standard or the enriched spike. An analysis of the sources of uncertainty suggests that the contribution from determination of the ratio is no longer dominant. Further improvements below 0.1% could be achieved by optimizing other sources of uncertainties, such as increasing the masses of enriched spike and natural abundance Mo standard solutions as well as the mass of high purity Mo standard used for its preparation, assuming similar measurement precision for the ratios and the same number of replicate samples measured.

 Please wait while we load your content...
Please wait while we load your content...