Osteoblast-derived paracrine factors regulate angiogenesis in response to mechanical stimulation
Abstract
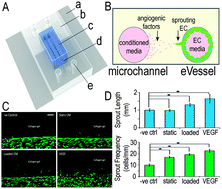
Angiogenesis is a process by which new blood vessels emerge from existing vessels through endothelial cell sprouting, migration, proliferation, and tubule formation. Angiogenesis during skeletal growth, homeostasis and repair is a complex and incompletely understood process. As the skeleton adapts to mechanical loading, we hypothesized that mechanical stimulation regulates “osteo–angio” crosstalk in the context of angiogenesis. We showed that conditioned media (CM) from osteoblasts exposed to fluid shear stress enhanced endothelial cell proliferation and migration, but not tubule formation, relative to CM from static cultures. Endothelial cell sprouting was studied using a dual-channel collagen gel-based microfluidic device that mimics vessel geometry. Static CM enhanced endothelial cell sprouting frequency, whereas loaded CM significantly enhanced both frequency and length. Both sprouting frequency and length were significantly enhanced in response to factors released from osteoblasts exposed to fluid shear stress in an adjacent channel. Osteoblasts released angiogenic factors, of which osteopontin, PDGF-AA, IGBP-2, MCP-1, and Pentraxin-3 were upregulated in response to mechanical loading. These data suggest that in vivo mechanical forces regulate angiogenesis in bone by modulating “osteo–angio” crosstalk through release of paracrine factors, which we term “osteokines”.


 Please wait while we load your content...
Please wait while we load your content...