Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1†
Abstract
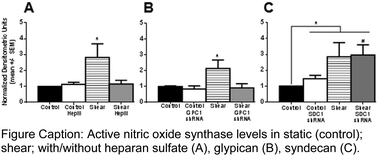
Mammalian epithelial cells are coated with a multifunctional surface glycocalyx (GCX). On vascular endothelial cells (EC), intact GCX is atheroprotective. It is degraded in many vascular diseases. GCX heparan sulfate (HS) is essential for healthy flow-induced EC nitric oxide (NO) release, elongation, and alignment. The HS core protein mechanisms involved in these processes are unknown. We hypothesized that the glypican-1 (GPC1) HS core protein mediates flow-induced EC NO synthase (eNOS) activation because GPC1 is anchored to caveolae where eNOS resides. We also hypothesized that the HS core protein syndecan-1 (SDC1) mediates flow-induced EC elongation and alignment because SDC1 is linked to the cytoskeleton which impacts cell shape. We tested our hypotheses by exposing EC monolayers treated with HS degrading heparinase III (HepIII), and monolayers with RNA-silenced GPC1, or SDC1, to 3 to 24 hours of physiological shear stress. Shear-conditioned EC with intact GCX exhibited characteristic eNOS activation in short-term flow conditions. After long-term exposure, EC with intact GCX were elongated and aligned in the direction of flow. HS removal and GPC1 inhibition, not SDC1 reduction, blocked shear-induced eNOS activation. EC remodeling in response to flow was attenuated by HS degradation and in the absence of SDC1, but preserved with GPC1 knockdown. These findings clearly demonstrate that HS is involved in both centralized and decentralized GCX-mediated mechanotransduction mechanisms, with GPC1 acting as a centralized mechanotransmission agent and SDC1 functioning in decentralized mechanotransmission. This foundational work demonstrates how EC can transform fluid shear forces into diverse biomolecular and biomechanical responses.

 Please wait while we load your content...
Please wait while we load your content...