Transforming potential and matrix stiffness co-regulate confinement sensitivity of tumor cell migration†
Abstract
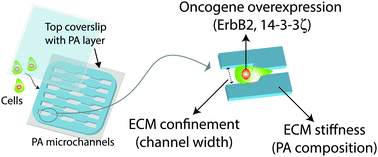
It is now well established that tumor cell invasion through tissue is strongly regulated by the microstructural and mechanical properties of the extracellular matrix (ECM). However, it remains unclear how these physical microenvironmental inputs are jointly processed with oncogenic lesions to drive invasion. In this study, we address this open question by combining a microfabricated polyacrylamide channel (μPAC) platform that enables independent control of ECM stiffness and confinement with an isogenically-matched breast tumor progression series in which the oncogenes ErbB2 and 14-3-3ζ are overexpressed independently or in tandem. We find that increasing channel confinement and overexpressing ErbB2 both promote cell migration to a similar degree when other parameters are kept constant. In contrast, 14-3-3ζ overexpression slows migration speed, and does so in a fashion that dwarfs effects of ECM confinement and stiffness. We also find that ECM stiffness dramatically enhances cell motility when combined with ErbB2 overexpression, demonstrating that biophysical cues and cell-intrinsic parameters promote cell invasion in an integrative manner. Morphometric analysis of cells inside the μPAC platform reveals that the rapid cell migration induced by narrow channels and ErbB2 overexpression are both accompanied by increased cell polarization. Disruption of this polarization occurs by pharmacological inhibition of Rac GTPase phenocopies 14-3-3ζ overexpression by reducing cell polarization and slowing migration. By systematically measuring migration speed as a function of

 Please wait while we load your content...
Please wait while we load your content...