A synthetic strategy for mimicking the extracellular matrix provides new insight about tumor cell migration†
Abstract
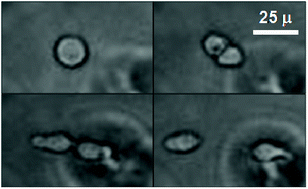
Understanding the role of the tumor microenvironment during cancer progression and metastasis is complicated by interactions between

 Please wait while we load your content...
Please wait while we load your content...