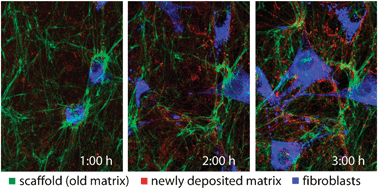
Elevated levels of tissue crosslinking are associated with numerous diseases (cancer stroma, organ fibrosis), and also eliminate the otherwise remarkable clinical successes of tissue-derived scaffolds, instead eliciting a foreign body reaction. Nevertheless, it is not well understood how the initial physical and biochemical properties of cellular microenvironments, stem cell niches, or of 3D tissue scaffolds guide the assembly and remodeling of new extracellular matrix (ECM) that is ultimately sensed by cells. Here, we incorporated FRET-based mechanical strain sensors, either into cell-derived ECM scaffolds or into the fibronectin (Fn) matrix assembled by reseeded fibroblasts, and demonstrated the following. Cell-generated tensile forces change the conformation of Fn in both 3D scaffolds and new matrix over time. The time course by which new matrix fibers are stretched by reseeded cells is accelerated by scaffold crosslinking. Importantly, stretching Fn fibers increases their elastic modulus (rigidity) and alters their biochemical display. Regulated by Fn fiber unfolding, more soluble Fn binds to the native than to the crosslinked scaffolds. Additionally, matrix assembly of fibroblasts is decreased by scaffold crosslinking. Taken together, scaffold crosslinking has a multifactorial impact on the microenvironment that reseeded cells assemble and respond to, with far-reaching implications for tissue engineering and disease physiology.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...