Solvent-free direct α-alkylation of ketones by alcohols catalyzed by nickel supported on silica–alumina†
Abstract
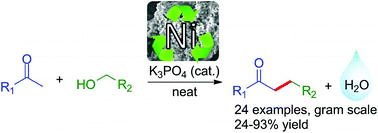
The α-alkylation of acetophenone with benzyl alcohol through borrowing hydrogen has been studied using nickel catalysis. Ni/SiO2-Al2O3 was found to be the best catalyst for this transformation and the corresponding alkylated acetophenone was obtained with 93% isolated yield. Following the objectives of clean and sustainable chemistry, the reaction occurs under solvent-free conditions and requires only a catalytic amount of base. This protocol was next applied to a wide range of ketones and alcohols and the desired products were isolated with 18–86% yields (26 examples). The recovery and recyclability of the nickel catalyst was also investigated and it was found to be active over 5 runs without significant loss of activity. Surprisingly, the active catalyst appears to include an amorphous nickel hydroxide layer.


 Please wait while we load your content...
Please wait while we load your content...