Transition-metal-free conversion of lignin model compounds to high-value aromatics: scope and chemoselectivity†
Abstract
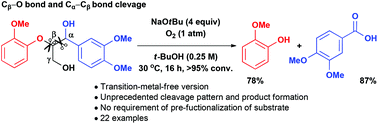
An efficient and straightforward reaction protocol for the conversion of lignin model compounds was developed based on a simple system consisting of a base, oxygen, and a green solvent under mild conditions in the absence of metals. This protocol was successfully applied to the cleavage of both ‘β-O-4’ dimeric and trimeric compounds, and a controlled selective degradation was achieved depending on the bond type. The feasibility of this method to provide aromatic compounds in high yields from lignin by a sequential oxidative dehomologation reaction was clearly demonstrated.


 Please wait while we load your content...
Please wait while we load your content...