Catalyst-free tandem halogenation/semipinacol rearrangement of allyl alcohols with sodium halide in water†
Abstract
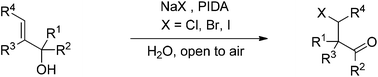
An economical, convenient and eco-friendly procedure for the one-pot synthesis of β-haloketones with an α-quaternary carbon center via the tandem halogenation/semipinacol rearrangement of allyl alcohols with NaX (X = Cl, Br, I) in H2O under open-air conditions has been demonstrated. The catalyst-free process affords various halogenated products in moderate to excellent yields (up to 98%). The features of this procedure include catalyst-free process, aqueous medium, low-cost sodium halide, open-air conditions, excellent yield and gram scalable preparation.


 Please wait while we load your content...
Please wait while we load your content...