Accelerated decarbonylation of 5-hydroxymethylfurfural in compressed carbon dioxide: a facile approach†
Abstract
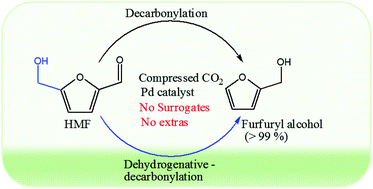
Herein, decarbonylation of biomass-based 5-hydroxymethylfurfural (HMF) in compressed CO2 with an unexpected acceleration of the reaction rate and excellent catalytic activity is reported. Without any additive, CO surrogates, or any organic solvents, via the developed method, an excellent conversion of 99.8% and highest selectivity of furfuryl alcohol (99.6%) in 4 h at 145 °C were achieved using an alumina-supported Pd catalyst (Pd/Al2O3). The superior activity is due to the unique characteristics (miscibility of reactant gases and high diffusivity) of compressed CO2 and the synergy between CO2 and Pd/Al2O3, where CO2 plays an interesting role in accelerating the reaction by enhancing the diffusion of CO and furfuryl alcohol (both products have high solubilities in CO2), consequently shifting the equilibrium to the forward direction. Characterisation of the catalyst suggested its direct interaction with the substrate and provided an indication of the possible reaction path. Thus, a mechanism was outlined. Compared to the results obtained using organic solvents, the results obtained using compressed CO2 were superior in terms of activity, selectivity, and reaction rate. This strategy highlights easy product separation, improved catalyst life, and a simple sustainable process. The efficiency of this protocol is confirmed by its potential application to a series of aldehydes with various substituents to produce decarbonylated products in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...
